Novel Non-Flushing Niacin Derivatives and Mimetics
SUMMARY
A researcher from the Tufts University School of Medicine has developed novel derivatives of Niacin, which do not produce the uncomfortable side effect of flushing and reddening of the skin, liver toxicity, or blood glucose changes in patients after high dose treatments.
Problem
Hyperlipidemia and hypercholesterolemia are conditions that are correlated with the increased risk of heart disease, stroke and death. Although there are numerous agents available for lowering blood lipid and cholesterol levels, like statins, these medicines do not improve the blood serum levels of the healthy lipoproteins, or high-density lipoproteins (HDLs). According to the American Heart Association, not only is reducing low-density lipoproteins (LDLs) levels essential to long-term heart health but improving HDL levels are just as important. Niacin, a water-soluble B-complex vitamin, has been used to accomplish both lowering LDLs and triglycerides, but also improve HDL levels. However, Niacin is known to cause skin flushing, and liver toxicity and blood glucose changes with long-term use. There is an urgent need to develop derivatives of Niacin that have a good tolerability and safety profile
SOLUTION
Novel Niacin analogues that have equal or greater HDL-raising ability while having minimal or no propensity to induce flushing or other side-effect from long-term use. As demonstrated through preclinical studies in multiple species, as well as clinical studies in healthy volunteers, demonstrate that high doses of the analogues do not lead to liver toxicity or blood glucose changes. Overall, the results support these analogues as a promising therapy for the management of mixed hyperlipidemia, particularly in the context of metabolic syndromes and type II diabetes.
DATA
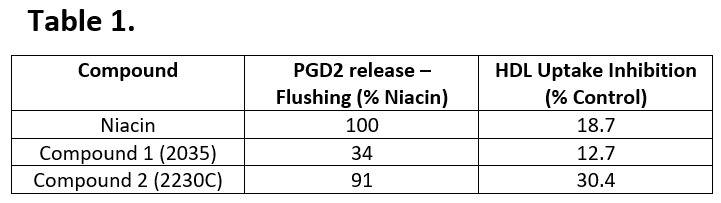
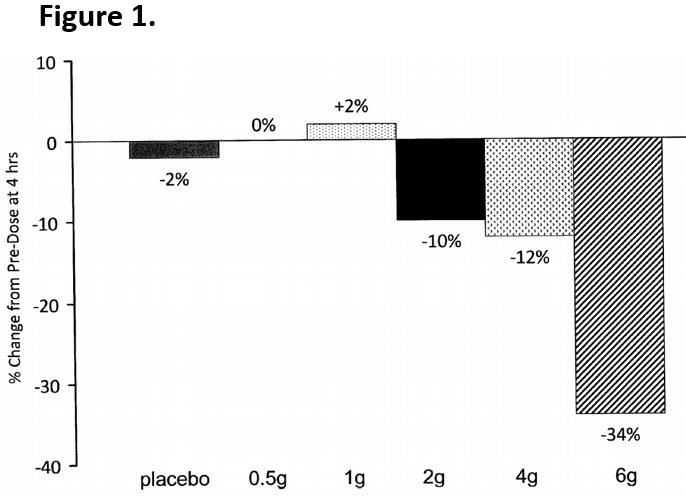
In a preclinical study of the analogues, a protein called PDG2 was assessed as it is associated with skin flushing (Table 1). The analogues display reduced flushing effects and equivalent or improved HDL uptake inhibition when compared to Niacin. Through two clinical studies (in healthy volunteers, the Niacin analogue was well tolerated with no flushing, no liver function abnormalities, and no blood glucose changes. In the group of patients receiving 3g BID, there was a 19% reduction in triglycerides, a 7.4% reduction in LDL, and an increase in HDL by 7.0%. After just a single dose of a 6gs of a Niacin derivative, patients experienced a 34% decline in triglyceride levels after just four hours (Figure 1).
IP STATUS
• T001396 - US patents: 8,377,971, 8,889,720, 9,193,708, and 9,511,060
• T001674 - US patents: 8,450,316, 8,937,063, and 9,212,142
Licensing Contact
Emma Anderson
EmmaMarie.Anderson@tufts.edu