New Neisseria gonorrhoeae Vaccine Antigens Expressed During Natural Mucosal Infection
SUMMARY
Researchers at Tufts University discovered novel antigens for a gonococcal vaccine through a unique candidate antigen selection strategy. In vivo, these previously unconsidered protein antigens induce a strong cross-reactive antibody response against diverse Gonorrhea strains
PROBLEM
Neisseria gonorrhoeae, a sexually transmitted infection, is a multi-faceted disease that causes severe reproductive tract complications in women. Gonorrhea has a high worldwide morbidity (87 million patients annually) and cases have increased by 63% since 2014 (CDC). Gonorrhea once was easily treated through intramuscular injections of antibiotics as the standard of care. However, there has been a recent emergence and spread of antibiotic-resistant N. gonorrhoeae strains. This rise of potentially untreatable infections has accelerated the search for an effective vaccine. Unfortunately, the pool of protective antigens from which to develop a vaccine remain scarce.
SOLUTION
Researchers implemented an innovative sample collection strategy with samples isolated from mucosal tissues of both infected male-female partners, as Gonorrhea affects men and women differently. This difference, and corresponding differing gene expression profile of N. gonorrhoeae strains in males vs. females, led to the Tufts researchers to select and study only the genes expressed across bacterial strains isolated from both sexes. This sex-based pool of antigens was narrowed even further to have the following properties: a) expression during actual infection of mucosal tissue, b) non-cytosolic, c) immunogenic, d) are hypothetical (i.e., gene sequences that exist but not previously known to be expressed), e) have mRNAs with relatively high expression, f) are conserved across different strains of N. gonorrhoeae, and g) have proposed functions based on sequence analogies. The 36 elucidated antigens have not previously been assessed as potential vaccine candidates.
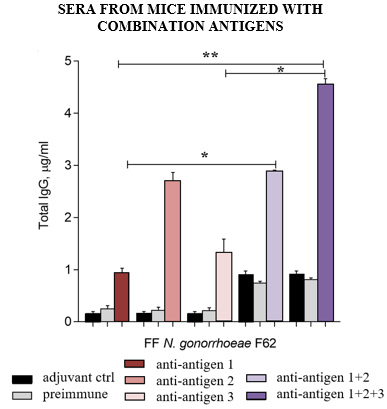
CURRENT DATA
Through an in vitro serum bactericidal assay, three of the identified antigens successfully induced the killing of N. gonorrhoeae strains. Further, flow cytometry supports that these three antigens are accessible to antibodies surface-exposed proteins on the bacterial surface across various strains. The researchers confirmed mouse antibody response specificity using N. gonorrhoeae deletion mutant strains. The antigens were then used to immunize mice with gonococcal vaginal colonization. In vivo these antigens demonstrated the ability to produce serum bactericidal activity and induce a cross-reactive antibody response against at least four N. gonorrhoeae strains. Pooled preimmune sera and immune sera were analyzed through ELISA for total antibody response against the FF N. gonorrhoeae F62 strain. The combination of all three antigens elicited a robust production of antigen-specific IgG in comparison to each antigen alone, the adjuvant control, and the preimmune sera (figure). These uniquely selected antigens have demonstrated efficacy for a novel multi-antigen universal vaccine the elicits broad and diverse protection against N. gonorrhoeae.
ADVANTAGES
- Selection based on gonococcal gene expression in natural infections across both sexes
- Serum bactericidal activity
- Cross-reactive antibody induction
- Surface-exposed
IP STATUS PCT filed in July 2020 (WO2021022001)
Licensing Contact
John Cosmopoulos
john.cosmopoulos@tufts.edu