Methodology to Engineer Amino Acid Ammonia Lyase Enzymes
SUMMARY
Tufts University investigator Nikhil Nair and his team have developed a novel directed evolution workflow to rapidly engineer ammonia lyase (AL) class enzymes for large sequences that are ideal for improving kinetic properties. This technique was used to isolate previously unexplored phenylalanine ammonia lyase (PAL) enzymes with ~2.5-fold higher activity compared to the active ingredient in the only approved drug for classical Phenylketonuria (PKU).
PROBLEM
PKU affects 1 in 15,000 in the US and can quickly progress to irreparable neurological and developmental defects, or death, in infants. The symptoms associated with PKU are caused by the build of phenylalanine in the blood due to an impaired phenylalanine hydroxylase enzyme. To date, PKU is managed mainly through lifelong dietary restriction of phenylalanine. Recently, PAL enzymes formulated for oral, injectable, or cell-based delivery have been investigated as therapeutics. However, the only approved treatment for classical PKU (Palynziq) has an FDA black box warning due to the likelihood of anaphylactic reactions and cannot be used in patients under the age of 18
SOLUTION
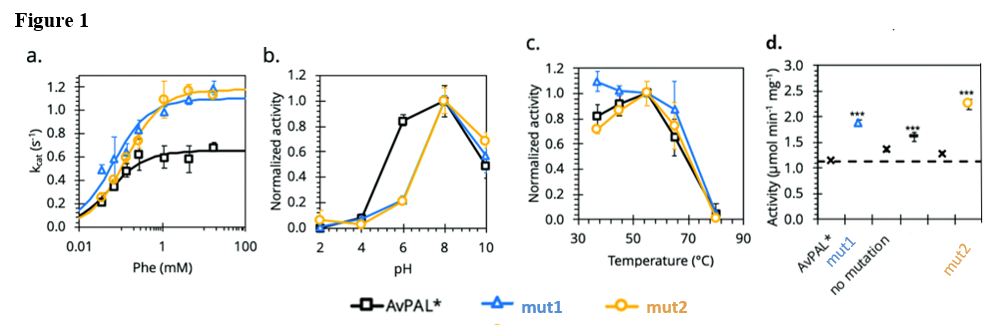
Through a rapid, directed evolution screening technique, that linked enzymatic activity to cell growth, highly active mutant PALs were selected for further analysis. The team developed a deep mutational analysis to explore the fitness landscape of a PAL mutant library and identified 79 mutational hotspots that increased enzyme fitness. The researchers then generated and enriched a semi-rational mutant library from a limited number of these hotspots and characterized the enzyme kinetics of a selected subset. All purified enzymes had enhanced kcat and Vmax, with multiple greater than 200% (Table 1). The improved kinetic parameters, and optimized pH, thermal, and proteolytic stability are highly advantageous for the therapeutic potential of these mutants.
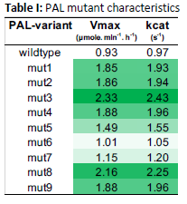
CURRENT DATA
The engineered enzymes demonstrate (a) 70-80% increase in PAL activity when compared to parental AvPAL*, (b and c) maintained optimal pH and temperature stability of the parental enzyme, and (d) a 1.5 to 2-fold higher PAL activity in comparison to other isolated colonies. PALs with increased enzymatic activity will enhance the therapeutic efficacy of current treatments, decrease the dosage quantity/frequency, which would decrease cost, increase availability, and decrease immune response (for injectable) of all current PAL enzyme replacement therapies
IP STATUS: PCT application filed February 2021.
Licensing Contact
Emma Anderson
EmmaMarie.Anderson@tufts.edu